Compressed air in a can.
This topic is locked from further discussion.
PV=nRT
Pressure x Volume = # of moles x Gas Constant (R) x Temperature.
If you're decreasing the left you have to decrease the right.
Perfect gas law. If pressure drops, and volume stays the same, then temperature has to drop.Dark__LinkNice job ruining a perfectly good thread in which we could make up funny reasons why the can gets cold. *shakes head*
[QUOTE="Dark__Link"]Perfect gas law. If pressure drops, and volume stays the same, then temperature has to drop.FragStainsNice job ruining a perfectly good thread in which we could make up funny reasons why the can gets cold. *shakes head* What?! I thought mine was a funny reason!! I just pulled something out of my ass, I thought it'd be hilarious! :(
PV=nRT
Pressure x Volume = # of moles x Gas Constant (R) x Temperature.
If you're decreasing the left you have to decrease the right.
uhoh_hotdogs
n is decreasing.
This has to do with the first law of thermodynamics.
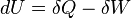
When you open the can, you are reducing the pressure and thus causing the gas to do work. This causes a decrease in heat energy and thus a decrease in temperature.
Of course I could be wrong, I haven't gone over this stuff in months.
[QUOTE="uhoh_hotdogs"]PV=nRT
Pressure x Volume = # of moles x Gas Constant (R) x Temperature.
If you're decreasing the left you have to decrease the right.
Oleg_Huzwog
n is decreasing.
Not by a significant enough amount it seems! :o[QUOTE="uhoh_hotdogs"]PV=nRT
Pressure x Volume = # of moles x Gas Constant (R) x Temperature.
If you're decreasing the left you have to decrease the right.
Oleg_Huzwog
n is decreasing.
Maybe it has to do with the fact that you're adding more moles of gas particles as well. From what I understand about aerosol cans, the liquid inside is volatile and this is what produces the gas. Pushing the valve open decreases the pressure of the system (the can) and allows the liquid to easily change into the gaseous phase (an increase in moles of gas even though some is lost out of the same valve). But I don't know, I could be wrong.
[QUOTE="Oleg_Huzwog"][QUOTE="uhoh_hotdogs"]PV=nRT
Pressure x Volume = # of moles x Gas Constant (R) x Temperature.
If you're decreasing the left you have to decrease the right.
uhoh_hotdogs
n is decreasing.
Maybe it has to do with the fact that you're adding more moles of gas particles as well. From what I understand about aerosol cans, the liquid inside volatile and this is what produces the gas. Pushing the valve open decreases the pressure of the system (the can) and allows the liquid to easily change into the gaseous phase (an increase in moles of gas even though some is lost out of the same valve). But I don't know, I could be wrong.
Ah, but that also means V of the gas is increasing as the liquid boils.
[QUOTE="uhoh_hotdogs"]Maybe it has to do with the fact that you're adding more moles of gas particles as well. From what I understand about aerosol cans, the liquid inside is volatile and this is what produces the gas. Pushing the valve open decreases the pressure of the system (the can) and allows the liquid to easily change into the gaseous phase (an increase in moles of gas even though some is lost out of the same valve). But I don't know, I could be wrong.
Oleg_Huzwog
Ah, but that also means V of the gas is increasing as the liquid boils.
I thought that since it's a gas, its volume is dictated by its container. The change is taking place inside the can, so wouldn't the volume would be considered (nearly) constant? Again, I have no idea, I forget things as soon as the exams are over.
This has to do with the first law of thermodynamics.
When you open the can, you are reducing the pressure and thus causing the gas to do work. This causes a decrease in heat energy and thus a decrease in temperature.
Of course I could be wrong, I haven't gone over this stuff in months.
the_one34
Yeah.....
What he said... o_0
[QUOTE="Oleg_Huzwog"][QUOTE="uhoh_hotdogs"]Maybe it has to do with the fact that you're adding more moles of gas particles as well. From what I understand about aerosol cans, the liquid inside volatile and this is what produces the gas. Pushing the valve open decreases the pressure of the system (the can) and allows the liquid to easily change into the gaseous phase (an increase in moles of gas even though some is lost out of the same valve). But I don't know, I could be wrong.
uhoh_hotdogs
Ah, but that also means V of the gas is increasing as the liquid boils.
I thought that since it's a gas, its volume is dictated by its container. The change is taking place inside the can, so wouldn't the volume would be considered (nearly) constant? Again, I have no idea, I forget things as soon as the exams are over.
Canned air starts in a liquid state inside the can. I was just responding to your statement of "adding more moles of gas" (which is correct, by the way), by pointing out that the volume occupied by the gas would be increasing as well. The total V remains the same, but the total n is decreasing.
This has to do with the first law of thermodynamics.
When you open the can, you are reducing the pressure and thus causing the gas to do work. This causes a decrease in heat energy and thus a decrease in temperature.
Of course I could be wrong, I haven't gone over this stuff in months.
the_one34
This is probably the only time you can apply that piece of wholly useless knowledge. :P
Canned air starts in a liquid state inside the can. I was just responding to your statement of "adding more moles of gas" (which is correct, by the way), by pointing out that the volume occupied by the gas would be increasing as well. The total V remains the same, but the total n is decreasing.
Oleg_Huzwog
Oh, I see. Then I don't know. The only thing I can think of then would be that the pressure decrease is disproportionately larger than the decrease in n, requiring the temperature to decrease as well. If that makes any sense.
Please Log In to post.
Log in to comment